Biodegradable polymers in biomedical applications: A review – developments, prospects and future challenges
Biodegradable polymers are a class of materials that are widely considered suitable for use in scientific fields such as tissue engineering and materials engineering due to their superior properties. Polymers in particular have attracted great interest in biomedical applications due to the alarming increase in the number of diseases and conditions diagnosed. The use of biodegradable polymers in biomedicine continues to expand. The application of new technologies or the improvement of existing technologies has made it possible to produce materials with desired properties, such as mechanical strength, controlled degradation times and rates, and antimicrobial and antibacterial properties. In addition, with proper design, these materials can assume almost unlimited shapes. This is also desirable when there is a need to develop new structures that support or restore the normal function of systems in the body.
I. Introduction
One of the fastest growing scientific fields is that related to biomaterials and their use. Intensive research is leading to the development of new generations of materials, the discovery of previously unknown properties, and the fabrication of biocomposites, which support medical scientists' work in therapy, diagnostics, and tissue regeneration more than ever before. In recent years, there has been a surge of interest in polymeric materials, especially those that are biodegradable. There is a growing confidence in polymers of both natural and synthetic origin. The high level of interest in this issue has led to an influx of research and increased our chances of getting new scientific reports. The use of biodegradable polymers is growing every year. This is confirmed by the number of emerging publications reporting on ongoing research. According to the Elsevier database (data as of October 20, 2023), the number of publications containing information on the manufacture, properties, and suitability of so-called "polymers for biomedical applications" is large. As shown in Figure 1, the use of biomaterials, nanostructures, or scaffolds is currently one of the hottest issues in medicine and healthcare.
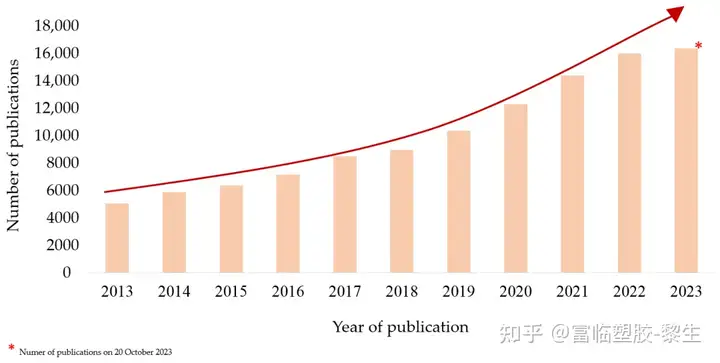
Figure 1. Data on the number of scientific papers published from 2013-2023 from the Elsevier database
As a preliminary statement, it is worth recalling the definition of biomaterials. Biomaterials are "intended to coexist with biological systems and are intended to treat, diagnose, correct or partially or completely replace tissues, organs or to perform their functions in vivo". Depending on the implantation site, the disease and its progression, the contact time of medical devices made of polymers with tissues may vary. The basic division of implant contact with the biological body includes three basic periods: short (transient), lasting up to 60 minutes; short-term contact, up to 30 days; long-term contact, lasting more than 30 days.
The standards for polymer materials dedicated to biomedical applications are very strict and are mainly driven by the safety of future users (patients). They have been standardized and included in the ISO 10993 standard. The standards include appropriate material selection, manufacturing processes, sterilization and effects on the body. All biomaterials applied in the biological environment must pass a series of biocompatibility tests. Implants are scaffolds that come into direct contact with blood, tissue, membranes or skin and are tested for: cytotoxicity, hemocompatibility, carcinogenicity, biodegradability, sensitization and reactivity with cells.
The advantages of biodegradable polymers over solid materials mainly include medical/clinical, economic and psychological benefits. For example, they do not require re-surgery to remove them from the body, compared to implants made of metal materials. Biodegradable polymers, whether natural or synthetic, are more biocompatible and more conducive to tissue regeneration than solid materials. Figure 2 shows the advantages of biodegradable polymers over non-biodegradable materials, considering the basic advantages.
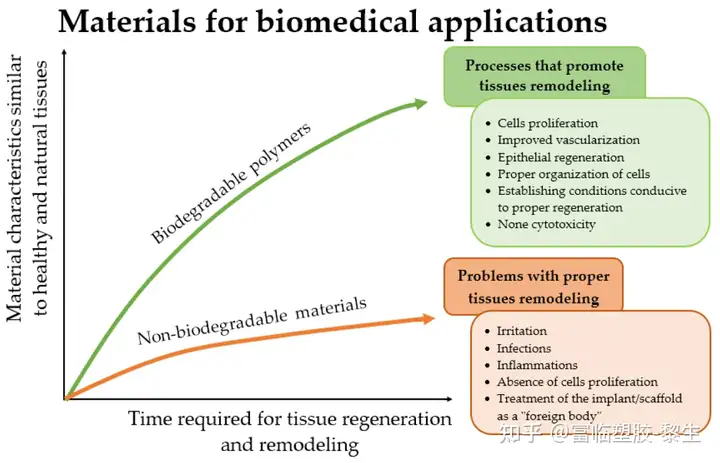
Figure 2. This figure illustrates the advantages of using biodegradable materials in biomedical applications compared to solid materials.
Before biomaterials based on natural or synthetic polymers are approved for internal use, detailed in vitro testing is required. These tests help select biomaterials with optimal biocompatibility and hemocompatibility under in vivo conditions.
Biodegradable polymers are used in a wide range of biomedical applications (Figure 3): tissue engineering and regenerative medicine, urology, controlled drug delivery systems, cardiac surgery, dentistry, orthopedics, and many more.
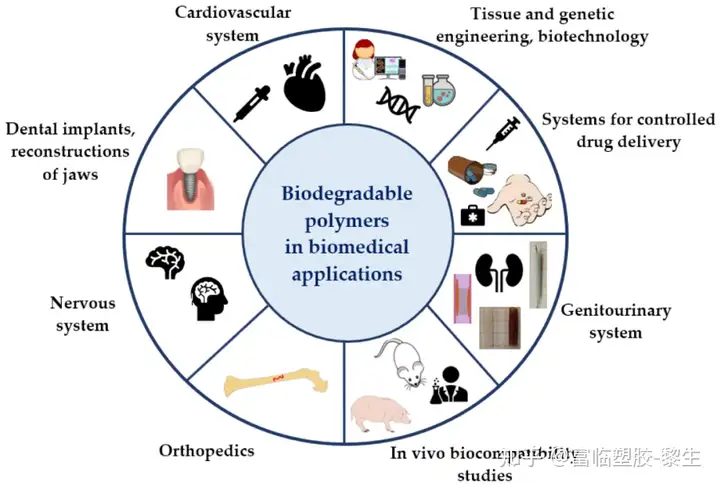
Figure 3. Applications of biodegradable polymers in biomedicine.
In this article, an attempt was made to characterize selected biodegradable polymer materials of natural and synthetic origin. Then, data on the methods of production of these materials and their frequency of use are presented and described. An important factor that should not be forgotten is the standards and requirements for biodegradable materials. Their understanding and subsequent application can determine the success or failure of a particular medical device. The manuscript also provides an overview of the applications of these materials in specific areas of biomedicine. What is presented in this article constitutes a small part of a large body of research conducted around the world.
II.Polymer-based biomaterials
Polymer materials can be divided into natural polymer materials and synthetic polymer materials. Despite their different origins, their functions are similar. Due to their applications in biomedicine, these materials should have certain properties. The basic properties of these materials are shown in Figure 4.
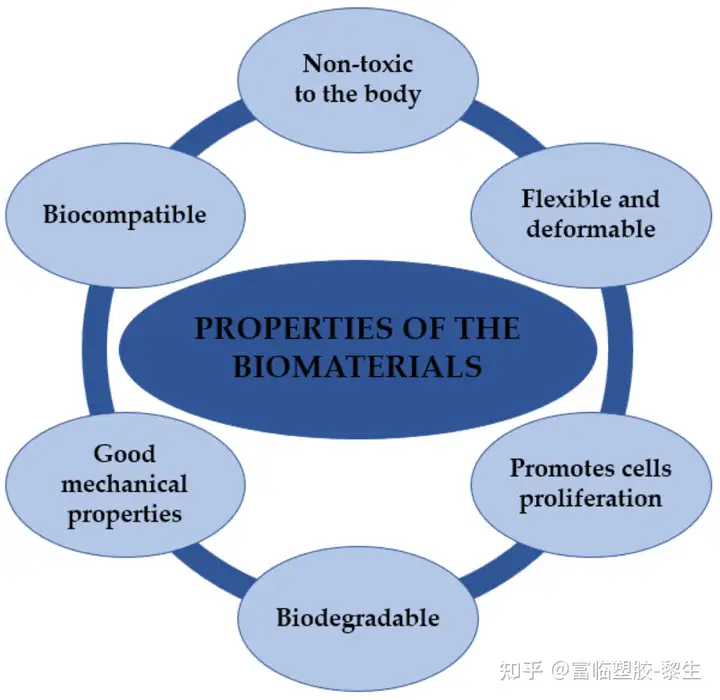
Figure 4. Characteristics of biomaterials based on natural and synthetic polymers.
Polymer materials are selected individually depending on the application. The choice of a given polymer is usually determined by its mechanical, material and biological parameters. Due to the diversity of these materials, the following subsections describe the characteristics of selected polymers from the natural and synthetic groups.
2.1. Natural polymers
Polymers of natural origin have attracted great interest in this research. Under natural conditions, they are produced by plants, animals or microorganisms. They are popular because of their fairly easy availability, low production cost and biocompatibility with living tissues. In addition, natural polymers are able to restore or maintain natural biological conditions, restore functions and provide structural support to the extracellular matrix (ECM). These are important characteristics to support healthy, functional interactions between tissues and implanted polymers. Stimulation of cell growth and differentiation processes promotes tissue regeneration. Although natural polymers have many advantages, it is necessary to mention their disadvantages. Due to the low origin and stability of the chemical structure of these materials, their strength and resistance to physicochemical stimuli are rather poor and low. It is difficult to produce multiple samples with consistent parameters and properties with natural polymers. The results of these materials are less reproducible, and manufacturing techniques such as sol-gel, although simple, do not give the same results. Natural polymers are widely used in regenerative medicine as dressing materials for difficult-to-heal wounds, cosmetics and controlled drug release systems.
2.1.1. Sodium alginate
Sodium alginate is a natural building block used to produce absorbable and bioactive hydrogels. Sodium alginate is an anionic hydrophilic polysaccharide belonging to the class of natural polymers. It is extracted from brown seaweed (Phaeophyceae). It consists of linear α-L-guluronic acid copolymers (G-blocks) and slightly branched and stretched β-D-mannuronic acid copolymers (M-blocks), which are linked by (1,4)-glycosidic bonds. The block arrangement in the structure of sodium alginate can appear in different configurations: fragments of GG blocks, fragments of MM blocks or in the form of their alternating MG arrangement. The structure of alginate hydrogels (enriched with a greater number of M blocks) is characterized by a slightly higher deformability than that of alginate gels, which contain mainly G blocks.
2.1.2. Chitosan
Chitosan is obtained by deacetylation of chitin. It is mainly extracted from crustaceans, such as shrimp and crab. In order to extract chitosan from chitin, it needs to undergo the above-mentioned deacetylation process. Chitin deacetylation can be carried out in a strongly alkaline environment or by enzymatic hydrolysis. Chitosan is a linear binary heteropolysaccharide made of glucosamine. Its chemical structure contains β-1,4-N-acetylglucosamine bonds. Scientists appreciate the properties of chitosan and bet on its use as a subcutaneous tissue scaffold in tissue engineering. Chitosan is highly biocompatible with tissues, biodegradable, non-toxic and, most importantly, has antimicrobial properties. It is slightly less used in drug delivery systems because it is difficult to dissolve in body fluids.
2.1.3. Collagen
One of the most well-known proteins in the human and animal body is collagen, which is a complex and large protein. It is an important structural component of the extracellular matrix (ECM) and accounts for one-third of all proteins in the body. It is found in structures such as skin, ligaments, cartilage and tendons. Collagen is formed from fibrous proteins containing a large number of amino acids. It is considered a cell scaffold. Collagen is involved in intercellular communication, supports the immunity of the organism and has very important functions related to immunity and the reception of mechanical stress stimuli. Collagen accounts for about 90% of the human body and is mainly found in the skin. Collagen has extremely valuable properties required for regenerative medicine and other biomedical applications. Collagen supports structural processes, cell growth, proliferation and migration. It is a biocompatible material that is biodegradable in the tissue environment and is non-cytotoxic to the body. It seems to be an ideal candidate for the rapid formation of tissue scaffolds.
2.1.4. Gelatin
Gelatin is a natural polymer; it is a protein isolated from animal collagen by hydrolysis. It is biocompatible and biodegradable. A large part of gelatin is water, so gelatin has a low mechanical strength. To increase its elasticity, additives in the form of other polymers or organic or inorganic components are most often used. Gelatin has an extremely high ability to absorb liquids. This condition promotes cell growth processes, which is the main task of regenerative medicine. Unfortunately, gelatin-based materials are characterized by poor stability, non-permanence, easy damage and sensitivity to changes in environmental conditions such as temperature. Currently, the main challenge is to optimize the composition of gelatin hydrogels to improve their stability and mechanical properties.
2.2. Synthetic polymers 2.2.1. Poly(L-lactide) - PLLA
Poly(L-lactide) (PLLA) is a synthetic homopolymer derived from plants (e.g. corn). It is a representative of the family of polylactide PLAs. PLLA has a semi-crystalline structure of about 30-40%. The biggest advantage of this polymer is that it can be obtained from renewable resources. It is currently considered one of the most promising biomaterials for biomedical applications. Compared with other synthetic polymers, PLLA is biodegradable, biocompatible, mechanically strong, has very good physical and chemical properties, and has low toxicity in the body and tissue environment.
2.2.2. Polydioxanone - PDO
Polydioxanone is a synthetic, fully absorbable polymer based on poly(ester-ether). PDO is a polymer synthesized from p-dioxanone monomers and is a semi-crystalline (crystallinity of about 55%), multi-unit repeatable ether-ester polymer, in which the ether group is responsible for the elastic chain network of the polymer. Polydioxanone is fully biodegradable. Due to its good mechanical properties, biocompatibility, low inflammatory response and being fully metabolized by the human body, it is believed that it may become a material for future biomedical applications.
2.2.3. Poly(lactic-glycolic acid)—PLGA
PLGA is a synthetic, biodegradable and biocompatible polymer obtained by ring-opening polymerization of lactide and glycolide. It is most commonly used to develop controlled drug release systems. The properties of PLGA can be designed in a controlled manner. They depend on the molar ratio between lactide and glycolide and the molecular weight of the polymer. PLGA appears to be a very good candidate for 3D structures. An increasing number of studies have shown that the application of PLGA in biomedicine may be promising. However, some limitations are emerging, indicating that PLGA can cause limited cell growth and adhesion; these may adversely affect the normal regeneration of soft or hard tissues.
2.2.4. Polycaprolactone—PCL
PCL is a synthetic, biodegradable polymer produced by ring-opening polymerization of the monomer ε-caprolactone. Polycaprolactone is a hydrophobic semicrystalline biopolymer. It has good solubility and has a low melting point. Due to its properties, it is considered a good component for polymer blends[61]. The average degradation time of PCL is relatively long, about 2-4 years. However, this time depends on the molecular weight of the polymer. The cleavage and decomposition of the ester chain leads to a rapid decrease in molecular weight. This process illustrates the mechanism of PCL degradation. In biomedicine, PCL is most commonly used in tissue engineering and as a component of controlled drug release systems.
III.Manufacturing techniques
In order to obtain polymer medical devices, it is necessary to develop or select existing manufacturing methods. It is simpler to manufacture single-component implants than to manufacture two-component or multi-component compounds. Combining them sometimes poses difficulties due to the chemical structure of polymers. Therefore, it is very important to thoroughly understand each polymer and its physicochemical properties, which will help in the selection of the material joining process. The finished medical implant with the desired geometry can be formed using a variety of methods. These techniques include sol-gel immersion method, electrospinning, 3D printing and bioprinting. These are currently the most popular methods used to develop and manufacture implants, stents, scaffolds or other parts, mainly for biomedical applications for internal use.
3.1. Sol-gel method
At this moment, it can be said that this is the oldest method selected in this manuscript. It is characterized by being very simple to use, versatility, and low cost of equipment and equipment operation. This method is widely used in biomedical applications related to inorganic blends and polymer synthesis. Sol-gel technology involves the transformation of liquid colloidal solutions into dense three-dimensional structures; in short, gels are obtained from sols. Ghedini et al. developed a system for the controlled release of drugs in the form of antibiotics using the sol-gel method. The use of this technology makes it possible to produce local drug delivery systems with antimicrobial activity and prevent infections in difficult-to-heal burn wounds. Tarlani et al. presented the controlled release of ibuprofen in alumina nanocomposites. Paramita et al. also used the sol-gel approach in their study. His goal was to develop a nanobioglass to promote bone tissue regeneration. The physicochemical characterization and biological responses of zinc-doped bioglass nanoparticles were performed. As the authors point out, the results obtained are promising. The use of sol-gel synthesis makes it possible to develop and produce nanobioglass/zinc, which can serve as a scaffold for biomolecules, is cytologically compatible, promotes cell proliferation and osteogenic differentiation. The use of this technology makes it possible to produce local drug delivery systems with antimicrobial activity and prevent infections in difficult-to-heal burn wounds. The goal of the study by Foroutan et al. was to obtain mesoporous structures that could become a good solution in the form of a controlled therapeutic ion transport system to support bone tissue regeneration.
3.2. Electrospinning
The past decade has shown that researchers are very interested in the work of developing and manufacturing nanofibers. Electrospinning is a technology that has contributed to the development of this field. It involves the production of nano- or micro-fibers through a process that exploits the principles of electrohydrodynamics. In the simplest terms, a strong electric field is applied to a liquid polymer (solution, emulsion) to form a polymer jet. The main advantages of electrospinning are high efficiency, relatively simple equipment operation, and cost-effectiveness. The structures produced by electrospinning are small in diameter, easy to modify, have a large specific surface area, and can be formed into a variety of shapes. The materials produced by this technology are successfully used in medicine as scaffolds, implants, or surfaces that promote the regeneration of pathologically altered tissues. Multilayered polylactide (PLA) nanofibers were manufactured using electrospinning with the drug cisplatin. The developed matrix is designed to prolong the release of therapeutic substances on surgical incisions and prevent local recurrence of cancer after surgical resection. The study was conducted on a mouse model. The authors state that the proposed solution can delay cancer recurrence, further prolong life, and is less toxic than previously used solutions. Flexible fibers composed of polycaprolactone (PCL) and polydimethylsiloxane (PDMS) were developed for bone tissue engineering applications. The flexible PCL/PDMS shape memory fibers proposed by the authors showed high biocompatibility in in vitro studies, promoted osteoblast proliferation and increased the expression of biomineralization. PLA/PVA nanofibers were developed and fabricated by coaxial electrospinning with the goal of tissue engineering applications. The formed nanofibers composed of PLA core and PVA shell had very good hydrophilicity, good mechanical properties and cytological compatibility, which successfully enabled their use in tissue regeneration. The electrospun nanofibers were also successfully used for regeneration in skin tissue engineering. Electrospun nanofibers based on chitosan and PVA were developed and further reinforced with halloysite nanotubes. The results showed that the proposed solution showed high biocompatibility, the developed structure was biocompatible, and the addition of halloysite nanotubes significantly increased the mechanical strength of the nanocomposite.
3.3. Three-dimensional (3D) printing
Additive manufacturing methods, also known as 3D printing, are advancing the scientific world; 3D technology involves the layer-by-layer deposition of materials. The end result is a three-dimensional shape of any design [79]. The 3D geometry based on a special code is developed using special software and then uploaded to the device. The technology can be divided into several types: selective laser sintering (SLS), stereolithography (SLA), inkjet printing or fused deposition modeling (FDM). One of the most popular methods is FDM. The main application areas of this technology are the manufacture of pharmaceutical products, tissue engineering scaffolds, tissue regeneration structures and implants for internal use. A composite fiber scaffold based on PLA polymer and BG bioactive glass was developed and manufactured using the FDM method. The scaffold was characterized by bioactivity and cytocompatibility. The presence of pores in the 3D structure promoted osteoinduction and provided good conditions for osteoblast differentiation and ingrowth of bone-forming cells located in the adjacent structure. Based on their research, the authors claim that the proposed PLA/BG 3D scaffold can be used for bone tissue engineering and presents a very interesting study. The researchers used 3D printing technology to produce nanocrystalline cellulose composites. The developed biomimetic scaffolds made of PLA, with the addition of cellulose natron (CNC) extracted from Ficus thonningii, showed good mechanical properties; they were found to be non-toxic to the body and biocompatible with bone cells. The proposed structural solution seems to be a good treatment approach for tissue engineering and regenerative medicine. Cardiac scaffolds based on PLA and PCL produced using FDM printing technology were developed. The manufactured scaffolds were tubular in shape. Their physicochemical properties were analyzed, including degradation time, mechanical properties, and whether the scaffolds exhibited and supported cell proliferation. The authors claim that the results obtained with the proposed design solution, the selected materials, and their manufacturing methods can successfully treat cardiovascular diseases. A three-dimensional scaffold for bone tissue regeneration based on PCL, PLGA, and hydroxyapatite was proposed. The authors believe that the combination of these materials can have a positive impact on the mechanical and biological properties of the printed structure. The scaffolds were mechanically and cellularly tested in the presence of bone marrow mesenchymal stem cells in a mouse model. The results showed that the addition of PCL increased the mechanical strength of the scaffold; PLGA promoted cell proliferation and adhesion to the scaffold; and hydroxyapatite enhanced the osteoblast formation process.
3.4. Bioprinting
3D bioprinting is the youngest and most personalized approach in biomedical applications. The combination of biomaterial science, tissue and organ anatomy and design, and bioprinting has revolutionized the medical world. 3D bioprinting technologies aim to create implants, replacement organs or tissues personalized for patients. The advantage of bioprinting over other technologies lies mainly in their ability to produce structures composed of biomaterials and cells simultaneously. Such scaffolds are almost identical to those found in the body. They exhibit similar mechanical, biological and structural properties, making them functional analogs of healthy organs. Given the increasing number of patients with organ problems, the huge number of planned transplants, and the significant problems with the availability of transplanted organs or tissues, bioprinting is an extremely valuable technology and the future of medicine.
3D bioprinting can be divided into four basic technologies: inkjet bioprinting, laser-assisted bioprinting, pressure-assisted bioprinting, and stereolithography. Using a so-called "bioprinting" printer requires depositing very small droplets of liquid directly onto a special cell culture dish or hydrogel structure. It is the most popular technology in 3D bioprinting and the cheapest. Laser-assisted bioprinting works by using laser energy to deposit materials directly onto a substrate. The components produced by this technology can have different sizes (from picoscale to nanoscale). The variability of these parameters depends on the biological properties of the biomaterials used, their rheological properties, their printing parameters, and the complexity of the geometry of the printed parts. Bioprinting works by forcing biomaterials (e.g., polymers, solutions) through a nozzle onto a fixed substrate using the pressure movement of a piston or a screw. The biomaterial extruded from the needle (usually in the micrometer range) is applied layer by layer to form the target 3D structure. In stereolithography, liquid materials (usually resins) are formed into a dense solid form based on the action of light. The advantage of stereolithography over other technologies is that it can produce parts with very high precision. The application of bioprinting in biomedicine is growing every year. A study was conducted to develop and optimize the bioprinting parameters of hydrogel materials based on sodium alginate and gelatin. The purpose of the tests was to obtain test protocols and results to ensure the optimal viability of osteoblast-like cells. Shokouhimehr et al. proposed three-dimensional hyperelastic bone scaffolds with antibacterial properties specifically for specific bone defects. The porous scaffolds containing iron oxide nanoparticles have been tested in vitro and in vivo in a rat animal model of significant bone loss in the femur. Their proposed solution can be used for regenerative treatments of bone tissue while reducing the risk of infection and contamination at the treatment site. Bioprinting was used to create 3D designs to treat large skeletal muscle defects. The developed implants have a high degree of structural integrity and promote muscle cell proliferation during tissue regeneration. The ability to create personalized 3D constructs that are structurally similar and, most importantly, able to mimic tissues and organs is undoubtedly the future of medical development for the next few generations.
IV.Requirements for polymers in biomedical applications
All polymer materials intended for internal use must meet certain, sometimes stringent, requirements. Depending on the application of the biomaterial, the requirements may vary. Biomaterials, in this case biopolymers, should have certain properties (Figure 5) that, firstly, will enable them to fulfill the required function and, secondly, not change under the influence of the changing conditions prevailing in the interaction with the body and in the environment. It, such as temperature, pressure, antigens or the action of X-rays or magnetic fields. All implants placed should not cause genetic changes and should not react with the blood in a way that would cause a change in its composition. Biodegradable polymers must not break down into products that are harmful to the body. They should not cause inflammation or infection or induce immunogenic reactions. The degradation time of the polymer should match and be sufficient to the time required for the regeneration and reconstruction of tissues and organs. The larger the polymer chain of the material, the longer the degradation time of the material.
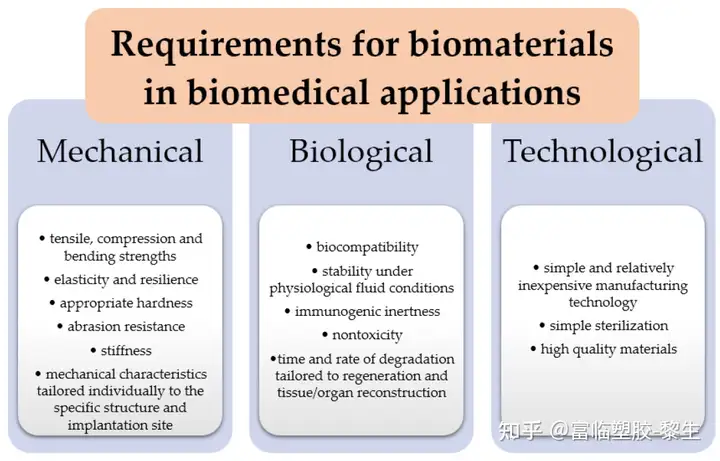
Figure 5. Requirements for biomaterials in biomedical applications.
The scientific community is still unable to eliminate the negative reactions of the human body to implants or medical devices. Many times, the body perceives implants as foreign objects that need to be expelled. Working on improving the biocompatibility of materials, their careful and professional selection, choosing appropriate manufacturing techniques, and using additional natural coatings can help and promote better implant-tissue integration. In the long run, this can successfully influence the possibility of manufacturing implants that completely replace malfunctioning organs. The correct integration of implants into the tissue environment and adaptation to tissue regeneration and remodeling processes can improve the function of the entire body.
V. Applications of biopolymers
Today, the use of polymers in biomedicine is already very widespread, and the increasing knowledge and understanding of these materials has promoted their use in many applications such as implants or prostheses. Both natural and synthetic polymers are used in tissue engineering, bone injury repair, urology, dermatology or neurology. The main function of the materials used is to provide a stable and temporary mechanical scaffold. The human body consists of different types of tissues. Each type has different structural properties, building blocks and physicochemical characteristics. Therefore, the structure, geometry and properties of the scaffold must be appropriately designed to meet these requirements. Scaffolds dedicated to bone tissue must first be resistant. This is due to the function of bones to provide stability and protection. Bones contain more extracellular matrix cells or collagen, which provide mechanical strength. In contrast, soft tissues - muscles, tendons and ligaments - contain more elastin to provide elasticity and flexibility, so scaffolds for these types of tissues should be more deformable and elastic.
5.1. Tissue Engineering
Injuries that occur in soft tissues can be caused by external mechanical factors, such as cuts or more complex injuries caused by related diseases. To provide conditions that are favorable for tissue regeneration and reconstruction, scaffolds/structures based on natural and/or synthetic polymers are designed and manufactured. Polymeric materials most often undergo hydrolytic degradation, producing natural products that are metabolized by the body. The time and rate of polymer degradation depends on the type used and can range from weeks to months or even years [98]. The use of polymer scaffolds in soft tissue tube regeneration combines several elements, such as the correct design and manufacture of the structure, collaboration with stem cells, and processes that promote cell growth. During the regeneration and reconstruction process, the damaged tissue requires structural support, which will further promote stem cell proliferation and migration.
5.2. Orthopedics - Bone Tissue Repair
Scaffolds specifically for bone tissue should promote osteoinduction and osteointegration, provide structural support and have very good mechanical properties. Implants used in orthopedics should provide temporary mechanical support to the affected bone defect site, promote the proliferation and growth of new cells leading to bone tissue reconstruction, promote appropriate cell ingrowth and adhesion to the porous scaffold, promote osteoinduction and local delivery of therapeutic substances to accelerate regeneration. For orthopedic scaffolds, the choice of the right polymer is very important. Therefore, it is necessary to carefully examine the properties of a given material to make it sufficient to meet the specific conditions of bone tissue. An improperly selected material that is too mechanically weak will not be able to perform the functions required for a proper healing process.
5.3. Urology
Urinary disorders have now become a major health problem worldwide. The number of diagnoses of urological disorders increases year by year. The most frequently diagnosed urological disorders include cancer, urethral strictures and ureteral obstruction. The task of urological reconstruction is to repair, regenerate and reconstruct part or all of the urinary system. Previously used treatments were to treat with natural resources such as transplanted tissue from the cheek or inside the small intestine, but today this is no longer sufficient. Therefore, intensive research is being conducted to produce alternatives in urology. Currently, the availability of polymers for this type of positioning is not a problem. Understanding the actual conditions inside the urinary tract remains a major challenge.
5.4. Neurology
An important system of any living organism is the nervous system. Damage to the nervous system caused by injury or disease can disrupt the functioning of the body and, in the worst case, can lead to death. The current availability of manufacturing methods with a wide range of polymer materials makes it possible to develop such implants, whose shape and properties will be able to support the treatment process. Both natural and synthetic polymers are used in neural tissue engineering, although currently the most trusted are those of natural origin. Polymer scaffolds help modulate biological signals, promote and guide axonal growth, slow or inhibit the formation of scar tissue. The future of polymers in treating CNS diseases is very interesting and interdisciplinary, but also challenging and not yet fully understood. The CNS is the most important system in the body, so it is important that treatments using polymer scaffolds are safe and clinically tested.
The development of regenerative medicine, biomaterials science, and the ability of medicine to conduct more and more research aimed at understanding function, disease, and its treatment. This is a very important aspect for humans worldwide. Table 1 lists selected examples of the applications of polymer materials in medicine.
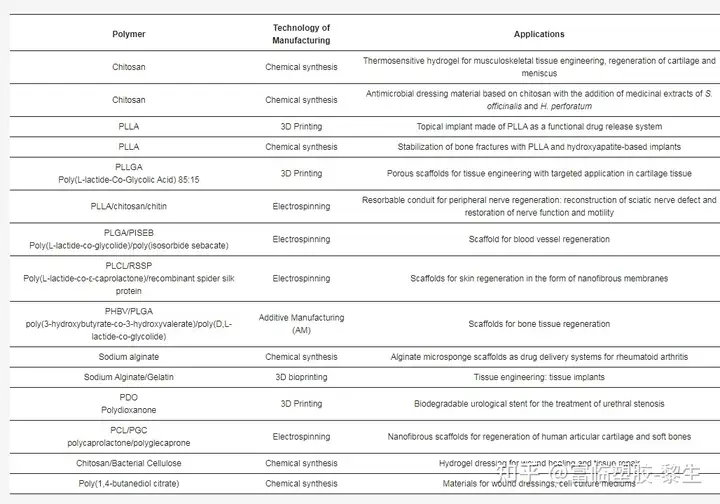
Table 1. Selected examples of polymer applications in biomedicine.
VI.Prospects for further research: challenges and limitations
The potential of natural and synthetic polymers for biomedical applications is enormous. A great deal of research has been conducted, providing us with valuable information about the structure, properties and potential functions of polymers. Scientists around the world are successfully conducting research on the suitability of polymer materials. Attempts are being made to produce hybrid polymer materials and characterize them using innovative equipment to image them and even identify their internal structure. Another important area of research is the development of polymer coatings for non-degradable materials. Such coatings are intended to improve the biotolerance and acceptance of the implant site. Surface modification of medical devices based on biodegradable polymers may help to treat various diseases (urinary system, circulatory system and difficult to heal wounds) more effectively. In order to safely apply the proposed solutions in the medical field, in-depth research on clinical needs is essential. Biodegradable polymer materials used in medicine, depending on the implantation site and use, are subjected to various external stimuli: pressure, flow, temperature, variable stress and strain, and electric fields. The materials used must be resistant to these stimuli and able to adapt to changing conditions. These variables seem to be the most difficult to understand and address. Undoubtedly, in recent years, there has been an explosive growth, success and breakthroughs in the manufacture of multifunctional biodegradable polymer materials. Further research should focus on chemical modification and the creation of hybrid polymer biocomposites that can mimic and regenerate bone and/or soft tissue.
The achievements and scale achieved here are impressive. The importance of continued development of these materials for medicine and patient treatment is clear. Interdisciplinary research combining tissue engineering, regenerative medicine, and biomaterials science is opening new avenues for future research. Although many successes have been achieved, many questions remain; these will continue into the future, revealing as yet undiscovered and perhaps even surprising questions and challenges. Further research must continue into enhancing the biocompatibility of biomaterials and their proper biological adaptation to the tissue environment. Issues of improving the body's response to implants and reducing inflammatory responses should be considered.
VII. Conclusion and discussion
This article reviews some polymer materials used in medicine. The research topics conducted here are very broad. The authors focus their attention only on some polymers and their specific areas of application. The challenge remains to select the right polymer for a specific use. Using a polymer that is too stiff and difficult to deform will not be suitable for implantation in hyperelastic tissues. However, using a polymer that is too easily deformed may not be able to unclog narrow arterial passages or urethra and ureters. It is necessary to deepen our knowledge of the interaction between biopolymers and tissue cells, physiological fluids and organs. In this regard, the continuous collaboration of many people is very important - physicians, biotechnologists and biomedical engineers.
The current development of the topic deserves a statement: this field is a powerhouse of science. Despite many successes, researchers still face many challenges and questions: How to improve the design, manufacturing and material selection of scaffolds dedicated to tissue engineering and regenerative medicine? The need for more scientific research work is mainly due to the current aging of the population and the emergence of a large number of diagnosed diseases and conditions, including new ones, whose causes and origins need to be understood and rediscovered.