Synthetic absorbable sutures are made from aliphatic absorbable polyesters containing one or more of five basic building blocks: glycolide, L-lactide, p-dioxanone, ε-caprolactone, and trimethylene carbonate. In addition to trimethylene carbonate building blocks, amorphous polymers are produced. The polymers produced by the other four building blocks are all semicrystalline.
The most important of these building blocks are glycolide and L-lactide, which are considered the "hard" segments, that is, the rigidity and flexibility are poor. ε-Caprolactone, p-dioxanone and trimethylene carbonate building blocks are considered “soft” segments that provide a more flexible suture fiber. Absorbable sutures made from 100% glycolide are absorbed most rapidly in the body, whereas absorbable sutures made from 100% or nearly 100% L-lactide or p-dioxanone take the longest to absorb.
The relationship between the molar percentage of glycolide in glycolide/L-lactide copolymer and the crystallinity of the copolymer
The relationship between the ratios is crucial in designing biomaterials for specific applications, such as Reed and Gilding (1979). Their data showed that glycolide-lactide copolymers must have greater than 70 mol% glycolide to achieve crystalline properties. For wound closure applications, synthetic absorbable sutures must have a certain degree of crystallinity. To achieve the appropriate tensile strength, which must be maintained during hydrolytic degradation. This is because biodegradable polymers with very low crystallinity or completely amorphous properties degrade very quickly, too fast to be used as a wound closure biomaterial. Therefore, a glycolide to L-lactide molar ratio of 90:10 has been used as the optimal comonomer ratio for suture use. However, it is well known that 100% pure L-lactide PLLA homopolymer degrades very slowly in vitro and in vivo due to its high crystallinity and hydrophobicity (from the pendant methyl groups), with a total absorption time reported to be more than 5.6 years.
Except for pure PDS or PDSII which only has the soft p-dioxanone structural unit, all commercial synthetic absorbable sutures all have at least one hard segment (glycolide or L-lactide). As shown in Table 11.1, suture manufacturers combine hard and soft structural elements to make flexible monofilament absorbable sutures and customize suture strength loss and mass absorption profiles.
In some cases, more than one soft structural unit is combined with a hard structural unit; but in all cases, the hard segment is always the major component (>50%).
Some examples are listed below:
•Monosyn has one hard segment (glycolide, 72%) and two soft segments (ε-caprolactone and trimethylene carbonate);
•Biosyn has a hard segment (glycolide, 60%) and two soft segments (p-Dioxanone and trimethylene carbonate);
•Both Maxon and Monocryl have a hard segment (glycolide, 67-75%) and one soft segment (32.5% trimethylene carbonate in Maxon, 25% ε-caprolactone in Monocryl);
•Caprosyn is the only synthetic absorbable monofilament suture that has two hard segments (L-lactide and L-lactide) and two soft segments (ε-caprolactone and trimethylene carbonate).
The chemical structures of these five basic building blocks and commercially available absorbable sutures are shown in Figures 11.1a and 11.1b, respectively. Recently, a 4-hydroxybutyrate-based absorbable suture (TephaFlex) received FDA approval. TephaFlex is made by bacterial bioengineering rather than traditional synthetic chemicals used to synthesize aliphatic absorbable polyesters.
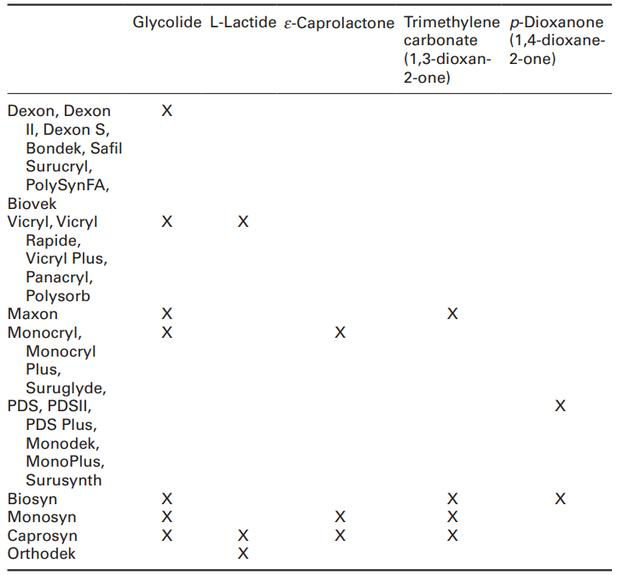
Table 11.1 Components of commercially available synthetic absorbable sutures.

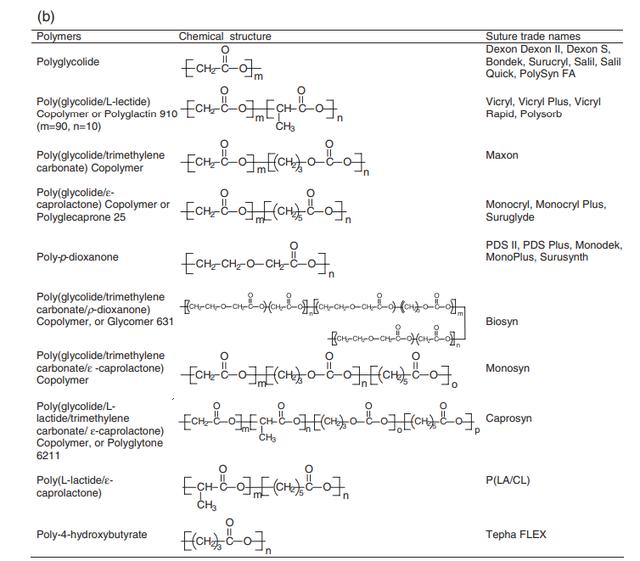
11.1 (a) Six components of synthetic absorbable sutures. (b) Chemical structure of commercial synthetic absorbable sutures.
If DL-lactide is used instead of L-lactide, the degradation rate is faster due to the amorphous nature of the resulting PDLLA. However, if DL-lactide is used as a comonomer with glycolide, the U-shaped relationship between crystallinity and glycolide composition observed by Reed and Gilding (1979) for glycolide/lactide copolymers disappears, since PLA composed of 100% DL-lactide is completely amorphous and degrades much faster than poly-L-lactide (PLLA).
Due to the high amorphous content, copolymer sutures made from glycolide and DL-rac-lactide can shrink up to 50%, while copolymer sutures made from pure D- or L-lactide isomers shrink very little. (1999) reported the use of DL-racemic polylactic acid (PDLLA) fibers as components of fiber-reinforced composite implants; the obtained PDLLA fibers have lower crystallinity, higher initial strength, and faster degradation than pure PLLA. At a composition ratio of 96 L and 4 D (PLA96), researchers (47/1995) demonstrated that DL-racemic PLA96 had a similar histological response to PLLA in rats, but degraded faster than PLLA due to significantly lower crystallinity and smaller degraded particle size. Similar results were reported by researchers (1995) in hydrolytic degradation studies of poly(DL-lactide) and by researchers (1999) in vitro and in vivo (rabbit) studies of self-reinforced PLA96 rods. Researchers (2001) reported that PLDLA sutures showed longer retention of tensile strength than Maxon sutures in vitro and in vivo, but had lower initial tensile strength than Maxon.
11.3.1 Polyglycolic acid (Dexon ®)
Polyglycolic acid (PGA) was the first synthetic absorbable suture introduced in the early 1970s (Frazza and Schmitt, 1971; Schmitt and Polistina, 1967; Katz and Turner, 1970). It was developed by Davis and Geck (now Covidien) under the trade name Dexon. There are many varieties of Dexon sutures. Dexon "S" is an uncoated PGA suture, while Dexon Plus and Dexon II have coating materials to facilitate handling, knot tying, and smooth passage through tissue. Dexon Plus is coated with poly(oxyethylene-oxypropylene) copolymer, while Dexon II is coated with a polycaproate coating. In addition to Covidien, other companies have introduced 100% pure PGA sutures, such as Medifit ® (Japan Medical Supplies Co., Ltd.), Safil and Safil Quick (Germany B. Braun AG), Bondek (Deknatel, USA), Surucryl (SURU International, India), Surgifit (AILEE, South Korea), and Biovek (Dynek, Australia).
PGA has an orthorhombic unit cell with dimensions a = 5.22 Å, b = 6.19 Å, and c (fiber axis) = 7.02 Å.
The close molecular packing and proximity of the ester groups are believed to stabilize the crystals. The specific gravity of PGA is 1.707 (perfect crystal) and 1.50 (completely amorphous material). The heat of fusion for 100% crystalline PGA is reported to be 12 KJ/mol (45.7 cal/g). Since PGA tends to be a rigid material, only multifilament braids or very fine monofilaments are suitable for suture use.
PGA for suture is polymerized from cyclic dimers of alpha-hydroxyacetic acid (commonly known as glycolic acid). PGA can be polymerized directly or indirectly from glycolic acid. For suture fiber grade PGA (i.e. molecular weight >20 000), ring-opening polymerization of glycolic acid cyclic dimers is used. There are many catalysts available for this ring-opening polymerization. They include organometallic compounds and Lewis acids. For biomedical applications, stannous chloride dihydrate or trialkylaluminum are preferred. If stannous chloride dihydrate is used (in the presence of alcohol), the polymerization mechanism is believed to be a cationic melt polymerization, and if trialkylaluminum is used, the polymerization mechanism is a nucleophilic attack of a carbanion on one of the glycolide carbonyl groups. Typically, an alcohol (such as lauryl alcohol) is added during the polymerization process to control the molecular weight. The resulting PGA polymers have an Mw of 20 000 to 140 000 and are suitable for fiber extrusion and suture fabrication.
Dexon suture fibers are made from melt spinning of PGA chips. The fibers are stretched to several hundred percent of their original length at a temperature above their glass transition temperature (approximately 36°C), heat set to improve dimensional stability and inhibit shrinkage, and then braided into the final multifilament braided suture form in a variety of sizes. Prior to packaging, all Dexon sutures are heated under vacuum to remove residual unreacted monomers or very low molecular weight volatile oligomers (Casey and Lewis, 1986; Glick and McPherson, 1967). Dexon sutures are sterilized using ethylene oxide because gamma irradiation is known to have the undesirable effect of accelerating the loss of tensile strength.
11.3.2 Poly(glycolide-L-lactide) copolymers (Vicryl ®、Vicryl Rapide ® 和 Polysorb ®)
Vicryl and Vicryl Rapide (Ethicon) and Polysorb (Covidien, formerly US Surgical, Tyco Healthcare, Mansfield, Mass.) are absorbable sutures made from glycolide-L-lactide random copolymers. Multifilament braided Vicryl sutures contain glycolic acid and L-lactic acid in a 90/10 molar ratio and are coated with 2–10% of a 50:50 mixtures of amorphous polylactic acid 370 (65/35 molar ratio of lactide-glycolic acid copolymer) and calcium stearate. Vicryl Rapide is a gamma-irradiated Vicryl that allows for more rapid absorption. It has been used for skin closure in young children because the sutures (which need to be removed before biodegradation) can be removed under local anesthesia rather than general anesthesia. It is also used in oral surgery.
Ethicon introduced a similar glycolide-L-lactide copolymer suture, Panacryl, in the late 1990s. They essentially reversed the glycolide to L-lactide molar ratio, setting the glycolide to L-lactide molar ratio to 5/95. Because the L-lactide component predominates in Panacryl suture, its absorption behavior is more like that of PLA than PGA, meaning that Panacryl takes at least 1.5 years or more to be completely absorbed in living tissue. Such a long in vivo absorption profile may be related to some of the reported postoperative complications. It is well known that prolonged in vivo degradation of absorbable sutures may put patients at risk for late tissue reactions, thus potentially offsetting the advantage of slow degradation. As a result, Ethicon withdrew Panacryl from the market in the mid-2000s.
11.3.3 Polydioxanone (PDS ®、PDS II ®、PDS Plus ® MonoPlus ®、Monodek ®、Surusynth ®)
Prior to the advent of PDS monofilament absorbable sutures, synthetic absorbable sutures were only available in large braided formats (Dexon and Vicryl) because the high density of ester functional groups made both of these sutures rigid in larger (e.g., 2/0) monofilament formats. There was a pressing need for a monofilament absorbable suture that was relatively large in size but also flexible for easier handling and better knot tying. This is because monofilament sutures generally have less tissue drag and tearing when passing through tissue and exhibit less tissue reaction than corresponding braided sutures. Monofilament sutures also do not provide capillary wicking, which can minimize the spread of wound infection, if any. One chemical approach to provide some molecular flexibility within the glycolide family is to reduce the density of ester functional groups in Dexon, which can reduce the frequency of hydrogen bonding. This strategy allowed the manufacture of monofilament absorbable sutures (such as PDS) with acceptable flexibility.
PDS and PDSII sutures were the first commercially available monofilament absorbable sutures from the glycolide family. They are referred to as poly(ester-ethers). These synthetic degradable monofilament sutures are polymerized from p-dioxanone monomers, 1,4-dioxanone-2,5-dione or p-dioxanone, the latter obtained by reacting chloroacetic acid with metallic sodium dissolved in a large excess of ethylene glycol. The resulting p-dioxanone is purified by multiple redistillations and crystallizations. Fiber-grade high molecular weight PDS polymers are prepared from highly purified (>99%) monomers by ring-opening polymerization in the presence of an organometallic catalyst such as Et 2 Zn or zirconium acetylacetonate. The resulting polymer has an intrinsic viscosity of 0.70 (0.1% polymer solution in tetrachloroethane at 25°C) and a crystallinity of 37%. The poly(p-dioxanone) polymers have a Tg = − 16°C to − 10°C and a Tm = 110–115°C.
Monofilament PDS sutures are made by melt spinning dried polymer chips through a spinneret into monofilaments of any desired suture diameter. The extruded fibers are then stretched approximately five times at T > 43°C and heat set to orient the molecules for better physical and mechanical properties. Pharmaceutical/cosmetic violet dye #2 is added to make purple PDS sutures. The 2–0 size PDS suture has an intrinsic viscosity of 0.64 and a crystallinity of 30%. PDS Plus is a PDS that has been surface treated with an antimicrobial agent, such as triclosan [5-chloro-2-(2,4-dichlorophenoxy) phenol], a potent broad-spectrum antibacterial and antifungal agent.
An enhanced version of PDS suture (PDSII) has been introduced that has improved flexibility and handling characteristics. PDS and PDSII are chemically identical but have different fiber morphologies due to different fiber spinning conditions. PDSII is made by briefly annealing melt-spun PDS fibers at a temperature above the PDS Tm (approximately 125°C) (Broyer, 1994). This additional heat-stretching treatment, which is not used in PDS sutures, partially melts the surface layer and subsequently alters the near-surface crystal structure of the monofilaments. As a result, a unique skin-core morphology is observed in PDSII sutures. The core of PDSII has a globular crystal structure that is more highly ordered and larger than the surrounding annular region, which is characterized by smaller crystals, whereas untreated PDS sutures display a relatively uniform crystal structure throughout their cross-section. In general, PDSII sutures have a relatively lower elastic modulus due to the presence of ether bonds and a reduced density of ester bonds. PDS sutures, like other synthetic absorbable sutures, are sterilized with ethylene oxide and are uncoated. Based on the chemical structure of PDS, i.e., ether and ester bonds separated by methylene (CH 2) groups, PDS is expected to be sensitive to degradation by oxidation, photooxidation, and gamma irradiation.
Several PDS-related copolymers have been reported in the literature that may have potential for use as wound closure biomaterials. Copolymers of PDS and PGA (20%) have similar absorption characteristics to Dexon and Vicryl sutures, but have compliance similar to PDS. Copolymers of PDS and PLLA (15%) produce sutures that are more compliant than homopolymer PDS, but have absorption characteristics similar to PDS.
11.3.4 Poly (glycolide-trimethylene carbonate) copolymer (Maxon ®)
Maxon absorbable suture is made of a copolymer of glycolide and trimethylene carbonate (1,3-dioxane-2-one) building blocks. Maxon consists of 32.5% (36 mol% by weight) trimethylene carbonate (Katz et al., 1985), which is a poly(ester-carbonate). The polymerization process is divided into two stages (Casey and Roby, 1982). The first stage is the formation of the midblock, which is a random copolymer of glycolide and 1,3-dioxane-2-one (trimethylene carbonate). Diethylene glycol is used as an initiator and stannous chloride dihydrate (SnCl 2 C∙2H 2 O) is used as a catalyst. The polymerization is carried out at about 180°C. The weight ratio of glycolide to trimethylene carbonate in the midblock is 15:85. After the mid-block synthesis, the temperature of the reaction bath is raised to approximately 220°C to prevent crystallization of the copolymer, and additional glycolide monomer is added to the reaction bath as an end block to form the final triblock copolymer. Undyed Maxon has a natural transparent appearance, while green Maxon is dyed with green DG#6 at less than 0.3% by weight. Maxon sutures are sterilized with ethylene oxide and no coating is used.
11.3.5 Poly (glycolic acid-co-ε-caprolactone) copolymer (Monocryl ®、Monocryl Plus ®、Suruglyde ®)
Monocryl, Monocryl Plus and Suruglyde absorbable monofilament sutures are made of copolymers with hard glycolic acid and soft ε-caprolactone building blocks. Monocryl Plus is an antimicrobial coated Monocryl, while Suruglyde is from SURU International. The copolymers have a wide range of lactone-based compositions, from as low as 15 to as high as 50 mol%, with weight-average molecular weights ranging from 4 510 to 16 500. The glass transition temperature ranges from 18 °C to − 43 °C, depending on the copolymer composition and molecular weight. The monofilament Monocryl suture has a composition of 75% glycolide and 25% ε-caprolactone and is a block copolymer consisting of a soft segment (ε-caprolactone) and a hard segment (glycolide). Monocryl is made using a two-stage polymerization process. In the first stage, the soft segment of glycolide and ε-caprolactone prepolymers is made. This soft segment prepolymer is further polymerized with glycolide to provide the hard segment of polyglycolide. The molecular weight of Monocryl should be higher than that of glycolide/ε-caprolactone copolymer reported by Fukuzaki et al. to satisfy adequate mechanical properties required for sutures.
The most important aspect of Monocryl monofilament suture is its flexibility, as claimed by Ethicon. For Monocryl, the force required to bend a 2/0 suture is only about 2.8 × 10 4 lb-in2 (193 MPa), while the same size of PDSII and Maxon monofilament sutures require about 3.9 and 11.6 × 10 4 lb-in2 (269–800 MPa), respectively. This inherent flexibility of Monocryl is attributed to its low glass transition temperature resulting from the ε-caprolactone comonomer unit. Its Tg is expected to be between 15°C and − 36°C. Unlike Maxon and PDSII, Monocryl suture appears to have less out-of-package memory, which improves its handling characteristics.
11.3.6 Poly (glycolide-trimethylene carbonate-dioxanone) triblock copolymer (Biosyn ®)
Biosyn ® absorbable suture is made of a triblock copolymer, Glycomer 631. It is composed of glycolide (60%), p-dioxanone (1,4-dioxanone, 14%), and trimethylene carbonate (1,3-dioxanone, 26%), with glycolide being the major component. The central block is a random copolymer of dioxanone (35% by weight) and trimethylene carbonate (65% by weight). Both ends of the central block are capped with block copolymers of glycolide (>50%) and p-dioxanone (<50%). Researchers have reported using gamma irradiation (2-12 Mrad doses) to shorten the absorption time of Biosyn (Roby and Arena, 1999). The use of gamma irradiation to reduce the absorption time of synthetic absorbable sutures is likely based on original studies by researchers in the 1980s and early 1990s on the effects of gamma irradiation on the degradation of synthetic absorbable sutures.
The purpose of introducing trimethylene carbonate into PGA when designing Maxon and Biosyn was to make synthetic absorbable monofilament sutures flexible without adversely affecting their other mechanical properties, such as knot strength and security, as well as their biodegradability. Sutures must be packaged with a bend, but must be able to be easily straightened when removed from the package prior to use and after a long shelf life. This ability to eliminate kinks is an important handling characteristic that surgeons always require when closing wounds.
Biosyn has the second-lowest elastic modulus of all existing synthetic absorbable suture materials (145 × 10 3 psi), and its strain energy at 5% and 10% strain (0.84 and 2.76 kg/mm, respectively) is approximately half that of a Maxon suture of the same size. Biosyn sutures lose tensile strength at a rate similar to that of Vicryl sutures, with only about 8% of their tensile strength remaining after 28 days in vitro. Biosyn has an in vivo absorption time of 90 to 110 days; however, absorption in rat skin takes up to 6 months. Of the three synthetic monofilament absorbable sutures tested by the researchers (Biosyn, Monocryl, and PDSII), Monocryl was completely absorbed within 90 days, Biosyn required 6 months to be nearly completely absorbed, and PDSII still had measurable mass after 6 months.
Biosyn's tissue response was between Monocryl (lower limit) and PDSII
(upper limit). For example, Monocryl, Biosyn, and PDSII had tissue response scores of 8, 20, and 28 at the end of 3 months, respectively. However, at the end of 6 months, the tissue score for Biosyn was very close to that for Monocryl (7 vs 5), while the score for PDS was 20, indicating that both Biosyn and Monocryl were essentially completely absorbed.
11.3.7 Poly(glycolide-co-trimethylene carbonate-co-ε-caprolactone) copolymer (Monosyn ®)
B. Monosyn absorbable monofilament suture from Braun AG is made of a triblock ABA-type terpolymer with three structural blocks: glycolide (72%), ε-caprolactone (14%), and trimethylene carbonate (14%) (Oberhoffner and Planck, 2000). ABA means that the B segment is capped at both ends by the A segment. A is the hard glycolide segment, while B is the soft segment, which is composed of glycolide, ε-caprolactone, and trimethylene carbonate block copolymers. Compared with Maxon, Monosyn has an additional third block, ε-caprolactone, which Maxon does not have. Compared with Biosyn, Monosyn has ε-caprolactone instead of p-dioxanone blocks. Compared with Monocryl, Monosyn has an additional third block: trimethylene carbonate (Table 11.1). Another major difference between Monosyn and Monocryl and Biosyn is that the soft segments in Monosyn have glycolide units, while neither Monocryl nor Biosyn have such units in their soft segments. B. Braun AG claims that the inclusion of some glycolide in the soft segments improves the compatibility between the hard and soft segments, thereby improving the mechanical properties of Monosyn (Oberhoffner and Planck, 2000). To achieve low modulus for better stitching flexibility, the soft segments must have 43 wt% or more. For example, the modulus values of fibers with 50 wt% and 40 wt% soft segments are 775 and 2012 N/mm2, respectively.
The triblock terpolymers were synthesized by conventional melt polymerization at 240°C in the presence of tin octoate catalyst and diethylene glycol initiator. Monosyn suture is made of ABA triblock terpolymer (glycolide/ε-caprolactone/trimethylene carbonate = 74/14/14) with 43 wt% soft segment (glycolide/ε-caprolactone/trimethylene carbonate = 35/32.5/32.5), intrinsic viscosity of 1.144 dL/g in HFIP solvent, melting point of 206.5°C, glass transition temperature of -19.1°C. Monosyn suture is melt spun into a water-cooled bath at ambient temperature and the spun Monosyn fibers are stretched at a draw ratio of 1:4 to 1:10 (Oberhoffner and Planck, 2000). The stretched Monosyn fibers are fixed by annealing at a temperature of 70°C to 130°C for 1 to 20 hours. The annealing process is used to provide a stable structure with the desired orientation.
The degradation of Monosyn sutures can be controlled by the total glycolide content and the soft segment weight percentage in the triblock terpolymer: an increase in the soft segment weight percentage accelerates degradation by decreasing crystallinity. For example, Monosyn fibers with 50 wt% soft segment and 70 wt% total glycolide content retained 44.2% and 7.4% of their nodule tensile breaking strength at 7 and 14 days in vitro, respectively, while Monosyn fibers with 43 wt% soft segment and 72 wt% total glycolide content retained 66.6% and 31.3% of their nodule tensile breaking strength at the corresponding times (Oberhoffner and Planck, 2000). Monosyn sutures lose approximately 50% of their original strength at 14 days in vivo and show no measurable strength at the end of 28 days after implantation. They take 60-90 days to be completely absorbed in the body, which is much shorter than the time required for other absorbable monofilament sutures, such as Biosyn (90-110 days), Monoacryl (90-120 days), PDSII (180-240 days), and Maxon (180-210 days).
11.3.8 Poly(glycolide-lactide-trimethylene carbonate-ε-caprolactone) copolymer or Polyglytone 6211 (Caprosyn ®)
Caprosyn is one of Covidien's newest synthetic absorbable monofilament sutures and the only synthetic absorbable suture made from Polyglytone 6211 copolymer, which has four structural units: glycolide, lactide, ε- Caprolactone and trimethylene carbonate.
Caprosyn retains 50% of its tensile strength at 5 days and is almost completely absorbed (intramuscularly) at 56 days, making it the fastest absorbing synthetic monofilament suture. Researchers (2004) showed that Caprosyn had a dermal tissue drag force of 54 ± 20 g and a stiffness (the amount of suture deflection at constant weight and suture length, i.e., greater suture deflection indicates lower stiffness) of 843 ± 106 × 10 –6 N/cm 2, significantly lower than chromium gut.
In a randomized trial of the appearance of tissue reaction in children (a 100 mm visual analog scale, or VAS score, used to assess scar outcome), researchers reported that after 13 weeks, the Caprosyn suture had a lower level of tissue reaction (VAS score of 83) is significantly lower than Vicryl Rapide suture (VAS score of 62). When comparing short-term (≤ 10 days) inflammatory responses to another synthetic absorbable monofilament suture (Monocryl) in the Wiscott adult male rat model, van Heerden (2005) reported that Caprosyn and Monocryl sutures were There was no statistically significant difference in the level of inflammatory response between the two groups, but Monocryl seemed to be more likely to cause allergic reactions, which was evident in the presence of mast cells and eosinophils. Researchers' (2007) randomized pilot study of skin closure after laparotomy for gynecological surgery further demonstrated similarities in clinical presentation and tissue response to Caprosyn and Monocryl.
11.3.9 Polylactic acid-based absorbable suture (Orthodek)
Although PLA has been used as a building block for glycolide-lactide based copolymer sutures such as Vicryl, 100% pure PLLA is not common as an absorbable suture, primarily due to its very slow absorption rate (5.6 years). The only commercially available PLA suture is Deknatel’s Orthodek: a coated braided PLA absorbable suture. Due to its very slow absorption rate, it is primarily used for tissues that require extended support, such as tendons, ligaments, and other tissues used for attachment to bone, but is not recommended for use in cardiovascular, ophthalmic, or neural tissues. In a November 2003 document, the FDA’s final determination found that Orthodek is similar to Ethicon’s Panacryl absorbable suture (95% L-lactide/5% glycolide) in terms of intended use, materials, design, and performance characteristics (FDA, 2003). There is no published data on Orthodek; however, it is generally believed to perform similarly to Panacryl as they have a 95% overlap in the L-lactide component.
11.3.10 Poly-4-hydroxybutyrate (TephaFlex)
The latest addition to the synthetic absorbable suture material is TephaFLEX, which is melt-spun from poly-4-hydroxybutyrate, which belongs to a class of absorbable biomaterials called polyhydroxyalkanoates or PHAs (FDA, 2007; Martin and Williams, 2003). Studies have shown that TephaFLEX is both biocompatible and non-inflammatory. Their biodegradation occurs through normal processes, and the degradation products are metabolites that are already present in the body.
Unlike other absorbable sutures on the market, P4HB is produced through a fermentation process rather than chemical synthesis (Martin and Williams, 2003). P4HB polyester is naturally produced within cells as storage particles that regulate energy metabolism. Therefore, it does not contain residual metal catalysts that can produce undesirable side effects such as inflammation.
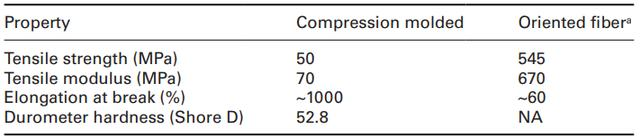
The production of P4HB is carried out through a proprietary genetically modified fermentation process in Escherichia coli, a microorganism that has become a workhorse in the biopharmaceutical industry. Using a fermentation process is advantageous not only because of the high yield (up to 50 g/L), but also because it allows the properties to be customized by incorporating other co-monomers and varying the molecular weight. Polymerization of 4HB with other hydroxy acids, such as 3-hydroxybutyrate (3HB), produces elastomeric compositions at moderate 4HB contents (20-35%) and relatively stiff rigid polyesters at lower 4HB contents (see Table 11.2) (Martin and Williams, 2003; Doi, 1990). In addition, the molecular weight of the polymer can be controlled by controlling the enzymes in the engineering pathway, allowing P4HB to have molecular weights as high as about one million with a polydispersity of 2-3 (Martin and Williams, 2003). Table 11.3 shows the mechanical properties of P4HB compared to currently synthesized absorbable biomaterials (Martin and Williams, 2003). P4HB has a Tg close to that of PCL, one of the lowest in the absorbable polymer class. P4HB is not as strong as PGA, but is more pliable and flexible than PGA and PLA. It has the potential to be stretched to 1000%, or ten times its original length, before breaking.
Table 11.2 Properties of absorbable elastic P4HB copolymers containing 3HB.
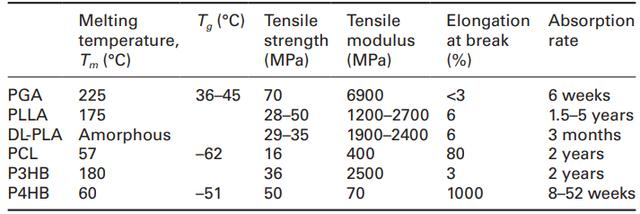
Table 11.3 Comparison of the properties of P4HB and other synthetic absorbable aliphatic polyesters.
Not only is P4HB biocompatible, it is indeed extremely well tolerated by the body. This is to be expected, as hydrolysis of P4HB produces 4HB, a naturally occurring metabolite found in the brain, heart, lungs, liver, kidneys, and muscles. Furthermore, this metabolite has a half-life of only 35 minutes and is rapidly eliminated by the body as carbon dioxide via the Krebs cycle. This is important because even if a product is biocompatible, it may still cause adverse tissue reactions due to accumulated high concentrations if the product is present in the body for a long time. Finally, polyesters have a low pKa (acid dissociation constant), making them less acidic than PGA and PLLA materials.
In the subcutaneous environment, P4HB appears to degrade more slowly than PGA, but faster than PLLA, polycaprolactone (PCL), and other polyhydroxyalkanoates such as poly-3-hydroxybutyrate (P3HB) (Table 11.3). (2000) reported that P4HB as a patch for the pulmonary artery of young sheep showed near-complete resorption histologically (169 days or 24 weeks) and that the porosity of the P4HB patch material (>95% porosity, 180-240 μm pore size) had a direct effect on the mass loss of the material in the subcutaneous environment. For example, it took 56 weeks for a 50% porous P4HB film to degrade in rat skin in vivo. The increased surface area did produce the expected result of increasing the degradation rate of the biomaterial. The mechanical changes caused by resorption were not abrupt but rather occurred gradually in P4HB, unlike those found in other synthetic absorbable polymers such as PGA. The gradual loss of strength is optimal for wound closure and healing because it allows time for the wound to adapt to the changes that occur. This suggests that degradation of P4HB is achieved by surface erosion rather than bulk degradation as is the case with PGA and PLA. Finally, P4HB is relatively stable to moisture, even during processing, and therefore has a good shelf life.
The mechanical properties and biocompatibility of P4HB make it a more suitable absorbable suture than currently available materials. The unique ability of the polymer to stretch its length nearly tenfold is due to the directional nature of the polymer chains, resulting in an exceptionally strong fiber. (Table 11.4) P4HB fibers are stronger than commonly used PP sutures (410-460 MPa) and are at least comparable in strength to commercially available absorbable materials such as Maxon (540-610 MPa) and PDS II (450-560 MPa). The primary differentiating factor between P4HB and typical sutures is handling during wound closure. The polymer has a lower Young's modulus, which equates to improved handling, and a different breaking strength retention curve when implanted. The Young's modulus of oriented P4HB fibers (670 MPa) is significantly lower than other monofilament sutures such as Maxon (2930 MPa), PDSII (1380 MPa), and Biosyn (1000 MPa). Table 11.4 gives a general overview of the mechanical properties of PH4B compared with other commonly used biomaterials.
Table 11.4 Mechanical properties of P4HB before and after.